

They described the substance to a meeting of the Imperial Institute of France. On 29 November 1813, Desormes and Clément made Courtois' discovery public. He also gave some of the substance to chemist Joseph Louis Gay-Lussac (1778–1850), and to physicist André-Marie Ampère (1775–1836). Ĭourtois gave samples to his friends, Charles Bernard Desormes (1777–1838) and Nicolas Clément (1779–1841), to continue research. Courtois suspected that this material was a new element but lacked funding to pursue it further. He noted that the vapour crystallised on cold surfaces, making dark crystals. Courtois once added excessive sulfuric acid and a cloud of purple vapour rose. The remaining waste was destroyed by adding sulfuric acid. To isolate the sodium carbonate, seaweed was burned and the ash washed with water. Saltpetre produced from French nitre beds required sodium carbonate, which could be isolated from seaweed collected on the coasts of Normandy and Brittany. At the time of the Napoleonic Wars, saltpetre was in great demand in France. In 1811, iodine was discovered by French chemist Bernard Courtois, who was born to a manufacturer of saltpetre (an essential component of gunpowder). It is on the World Health Organization's List of Essential Medicines. Iodine is also used as a catalyst in the industrial production of acetic acid and some polymers. Because of the specificity of its uptake by the human body, radioactive isotopes of iodine can also be used to treat thyroid cancer.
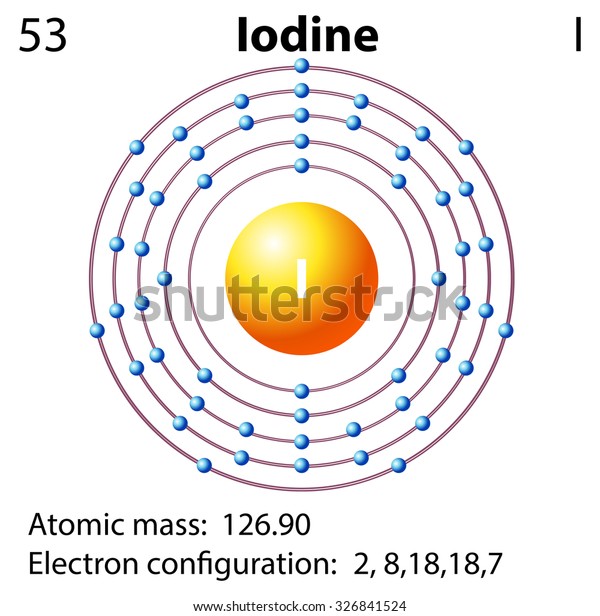
Due to its high atomic number and ease of attachment to organic compounds, it has also found favour as a non-toxic radiocontrast material. The dominant producers of iodine today are Chile and Japan. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. It is the least abundant of the stable halogens, being the sixty-first most abundant element. Iodine occurs in many oxidation states, including iodide (I −), iodate ( IO −ģ), and the various periodate anions. The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek Ιώδης 'violet-coloured'. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at 114 ☌ (237 ☏), and boils to a violet gas at 184 ☌ (363 ☏). Iodine is a chemical element with the symbol I and atomic number 53.


 0 kommentar(er)
0 kommentar(er)
